Answer:
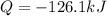
Step-by-step explanation:
Hello,
In this case, by means of the released heat, we need to consider the cooling of water in two steps:
1. Condensation of steam at 100 °C.
2. Cooling of water from 100 °C to 37 °C.
Therefore, we need the enthalpy of condensation of water that is 40.65 2258.33 J/g and the specific heat that is 4.18 J/g°C for the same amount of cooled water to obtain:
![Q=50.0g*[-2258.33(J)/(g)+4.18(J)/(g\°C)(37-100)\°C]\\\\Q=-126.1kJ](https://img.qammunity.org/2021/formulas/chemistry/college/b5gk9myfjmny9fnji13vvijm1f3yvwars2.png)
Best regards.