Answer : The balanced chemical equation will be:
(i)
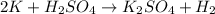
(ii)
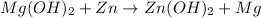
Explanation :
Balanced chemical equation : It is defined as the equation in which total number of individual atoms on the reactant side is equal to the total number of individual atoms on product side.
Part (i):
The balanced chemical equation will be:
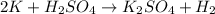
This reaction is a single displacement reaction in which most reactive element (potassium) displaces the least reactive element (hydrogen) form their solution.
Part (ii):
The balanced chemical equation will be:
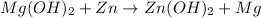
This reaction is a single displacement reaction in which most reactive element (zinc) displaces the least reactive element (magnesium) form their solution.