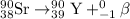Answer : The chemical equation for the beta decay process of
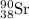 follows:
follows:
Explanation :
Beta decay : It is defined as the process in which beta particle is emitted. In this process, a neutron gets converted to a proton and an electron.
The released beta particle is also known as electron.
The beta decay reaction is:
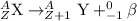
The chemical equation for the beta decay process of
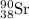 follows:
follows: