Answer:
(a)
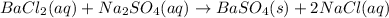
(b) The particles of the formed barium sulfate remain suspended in the aqueous media forming a colloid.
Step-by-step explanation:
Hello,
In this case, when solutions of barium chloride and sodium sulfate are mixed, the following chemical reaction is carried out:
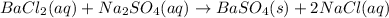
Thus, we can notice that the product barium sulfate remains solid since its solubility in aqueous media is very low, for that reason at the beginning the solution becomes cloudy as its particles remain suspended in the water forming a colloid. Nevertheless, after some days, the suspended particles get precipitated by the effect of the gravity, therefore, we observe the solid on the bottom of the beaker.
Regards.