Answer:
The thermal efficiency,
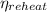 , of the Rankine cycle with reheat is 36.81%
, of the Rankine cycle with reheat is 36.81%
Step-by-step explanation:
p₁ = 8 MPa = 80 Bars
T₁ = 450°C = 723.15 K
From steam tables, we have;
v₁ = 0.0381970 m³/kg
h₁ = 3273.23 kJ/kg
s₁ = 6.5577 kJ/(kg·K) = s₂
The p₂ = 0.8 MPa
T₂ = Saturation temperature at 0.8 MPa = 170.414°C = 443.564 K
h₂ = 2768.30 kJ/kg
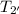 = 400°C = 673.15 K
= 400°C = 673.15 K
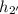 = at 400°C and 0.8 MPa = 3480.6 kJ/kg
= at 400°C and 0.8 MPa = 3480.6 kJ/kg
p₃ = 10 kPa = 0.1 Bar
T₃ = Saturation temperature at 10 kPa = 45.805 °C = 318.955 K
h₃ = 2583.89 kJ/kg
h₄ =
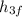 = 191.812 kJ/kg
= 191.812 kJ/kg
The thermal efficiency,
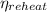 , of a Rankine cycle with reheat is given as follows;
, of a Rankine cycle with reheat is given as follows;
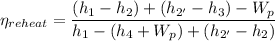
Therefore, we have;
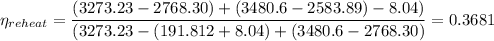
Which in percentage is 36.81%.