Answer:
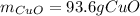
Step-by-step explanation:
Hello,
In this case, the undergoing chemical reaction is:
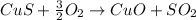
Thus, given the 1.00-kg of 12.5% ore, we can compute the theoretical yield of copper (II) oxide via stoichiometry:
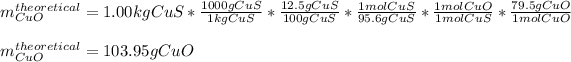
Whereas the third factor accounts for the percent purity of the covellite. Then, given the percent yield, we can compute the actual yield by:
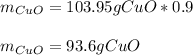
Regards.