Answer:
m = 50.74 kg
Step-by-step explanation:
We have,
Initial temperature of water is 20 degrees Celsius
Final temperature of water is 46.6 degrees Celsius
Heat absorbed is 5650 J
It is required to find the mass of the sample. The heat absorbed is given by the formula ad follows :
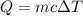
c is specific heat of water, c = 4.186 J/g°C
So,
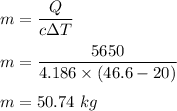
So, the mass of the sample is 50.74 kg.