Answer:
U = 12,205.5 J
Step-by-step explanation:
In order to calculate the internal energy of an ideal gas, you take into account the following formula:
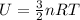 (1)
(1)
U: internal energy
R: ideal gas constant = 8.135 J(mol.K)
n: number of moles = 10 mol
T: temperature of the gas = 100K
You replace the values of the parameters in the equation (1):
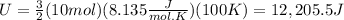
The total internal energy of 10 mol of Oxygen at 100K is 12,205.5 J