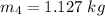Answer:
The mass of the lead will be "1.127 kg".
Step-by-step explanation:
The given values are:
(Ice) m₁ = 50 g i.e.,
0.050 kg
(Water) m₂ = 195 g i.e.,
0.190 kg
(Copper cup) m₃ = 100 g i.e.,
0.100 kg
m₁, m₂ and m₃ at temperature,
t₁ = 0°C
Temperature of lead,
t₂ = 96°C
Temperature of Final equilibrium,
t₃ = 12°C
Let m₄ be the mass of the lead.
On applying formula, we get
⇒
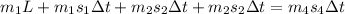
On putting the estimated values, we get
⇒
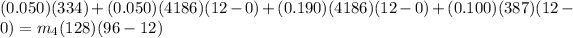
⇒
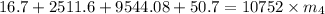
⇒
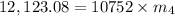
⇒
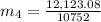
⇒