Answer:
The conversion in the real reactor is = 88%
Step-by-step explanation:
conversion = 98% = 0.98
process rate = 0.03 m^3/s
length of reactor = 3 m
cross sectional area of reactor = 25 dm^2
pulse tracer test results on the reactor :
mean residence time ( tm) = 10 s and variance (∝2) = 65 s^2
note: space time (t) =
t =
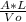 Vo = flow metric flow rate , L = length of reactor , A = cross sectional area of the reactor
Vo = flow metric flow rate , L = length of reactor , A = cross sectional area of the reactor
therefore (t) =
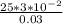 = 25 s
= 25 s
since the reaction is in first order
X = 1 -

 = 1 - X
= 1 - X
kt = In
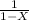
k = In
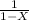 / t
/ t
X = 98% = 0.98 (conversion in PFR ) insert the value into the above equation then
K = 0.156
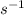
Calculating Da for a closed vessel
; Da = tk
= 25 * 0.156 = 3.9
calculate Peclet number Per using this equation
0.65 =
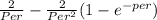
therefore
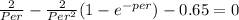
solving the Non-linear equation above( Per = 1.5 )
Attached is the Remaining part of the solution