Answer:
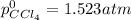
Step-by-step explanation:
Hello,
In this case, the undergoing chemical reaction is:
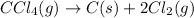
Therefore, the law of mass action is:
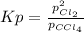
Pressure of carbon at equilibrium is not considered since it is solid. In such a way, based on the law of mass action, at equilibrium we have:
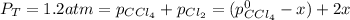
And in the law of mass action:
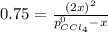
Now, from the total pressure, we have that:
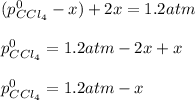
And we combine:
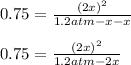
Thus, solving for
 we have to roots:
we have to roots:
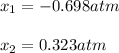
Clearly, the solution must be 0.323 atm, in such a way, the initial pressure turns out:
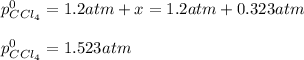
Regards.