Answer and Explanation:
The computation of the energy or wavelength gamma rays observed is shown below:
Since the energy of gamma rays is higher than 0.10 MeV.
Now We have to calculate transitions in between the given levels of energy that correspond to this energy.
As per the given question, we have the following information
Ground state = E where E < 0.075 MeV
For Level 1 = 0.075 MeV
For Level 2 = 0.320 MeV
For Level 3 = 0.475 MeV
Now we have to take the below transitions:
1.

Difference of energy is
= 0.475 - 0.320
= 0.155 MeV
This represents a gamma radiation
2.
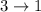
Difference of energy is
= 0.475 - 0.075
= 0.4 MeV
This represents a gamma radiation
3.
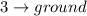
Difference of energy is
= 0.475 - E > 0.155 MeV
This represents a gamma radiation
4.
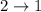
Difference of energy is
= 0.320 - 0.075
= 0.245 MeV
This represents a gamma radiation
5.
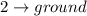
Difference of energy is
= 0.320 - E > 0.245 MeV
This represents a gamma radiation
6.
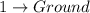
Difference of energy is
= 0.075 - E < 0.10 MeV
This represents not a gamma radiation
We can see that there are 5 transitions that contain gamma rays