Answer:
(a)
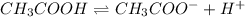 : reverse reaction is favored.
: reverse reaction is favored.
(b)
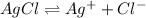 : reverse reaction is favored.
: reverse reaction is favored.
(c)
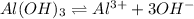 : reverse reaction is favored.
: reverse reaction is favored.
(d)
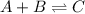 : forward reaction is favored.
: forward reaction is favored.
Step-by-step explanation:
Hello,
(a)
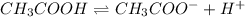 :
:
In this case, since the Ka is lower than 1, we infer the reverse reaction is favored since the reactant (acetic acid) will tend to have a higher concentration.
(b)
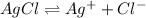 :
:
In this case, since the Ka is lower than 1, we infer the reverse reaction is favored since the reactant (silver chloride) will tend to have a higher concentration.
(c)
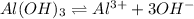
In this case, since the Ka is lower than 1, we infer the reverse reaction is favored since the reactant (aluminium hydroxide) will tend to have a higher concentration.
(d)
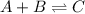
In this case, since the Ka is greater than 1, we infer the forward reaction is favored since the product (C) will tend to have a higher concentration.
Regards.