Answer:
Physical states with molecular equation:
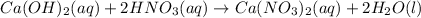
Net ionic equation:
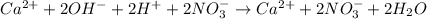
Step-by-step explanation:
Hello,
In this case, we can write the molecular equation and the physical states of the compound as shown below:
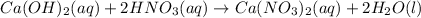
Thus, we notice that calcium hydroxide, nitric acid and calcium nitrate remain aqueous since the reaction is carried out in water as the media, therefore, also, water remains in liquid state.
Moreover, the net ionic equation is shown by expressing all the involved compounds in ionic form:
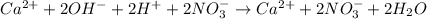
Thus, we notice that we can simplify it since calcium and nitrate ions are being spectator ions, so the remaining substances in the net ionic equation are:
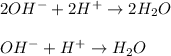
Best regards.