Answer:
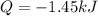
Step-by-step explanation:
Hello,
In this case, given the neutralization reaction between sodium hydroxide and hydrochloric acid:

We can compute the heat that is produced due to the reaction by noticing that the hydrochloric acid is the limiting reactant, so the change in the enthalpy of reaction is referred to it. In such a way, we compute the reacting moles of acid:
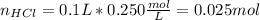
Finally, we compute the heat by using the given enthalpy of reaction:
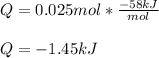
Best regards.