Answer:
![Keq=([CH_3OH])/([CO][H_2]^2)](https://img.qammunity.org/2021/formulas/chemistry/college/ydbahv1zo1k5ck91lsfluspvyos6698nze.png)
Step-by-step explanation:
Hello,
In this case, for the described reaction at equilibrium:
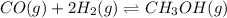
The analysis of the law of mass action allows us to write the equilibrium expression as shown below:
![Keq=([CH_3OH])/([CO][H_2]^2)](https://img.qammunity.org/2021/formulas/chemistry/college/ydbahv1zo1k5ck91lsfluspvyos6698nze.png)
Which is written considering that carbon monoxide, hydrogen and methanol are all in gaseous phase, for that reason all of them are included in the expression due to homogeneous equilibrium. Moreover, since hydrogen has a stoichiometric coefficient of 2, it is squared in the law of mass action.
Best regards.