Answer: K e q equals StartFraction StartBracket upper C EndBracket superscript lower c StartBracket upper D EndBracket superscript lower D over StartBracket upper A EndBracket superscript lower a StartBracket upper B EndBracket superscript lower b EndFraction.
Step-by-step explanation:
Equilibrium constant is the ratio of the concentration of products to the concentration of reactants each term raised to its stochiometric coefficients. It is represented by the symbol
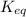
The balanced chemical reaction is:
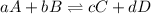
The expression for
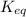 is written as:
is written as:
![K_(eq)=([C]^c* [D]^d)/([A]^a* [B]^b)](https://img.qammunity.org/2021/formulas/chemistry/college/4mjma7eve7x0g2ll5a4e6ceyjowi1j0lki.png)
Thus the correct option is K e q equals StartFraction StartBracket upper C EndBracket superscript lower c StartBracket upper D EndBracket superscript lower D over StartBracket upper A EndBracket superscript lower a StartBracket upper B EndBracket superscript lower b EndFraction.