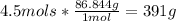Answer: 391 g
Step-by-step explanation:
For this problem, we need to know that molarity is. Molarity is moles of solute/liters of solution. it is also denoted as M=n/V, which is also mol/L. We are given that the molarity is 3.0 M and the liter is 1.5 L. All we have to do is plug in 3.0 and 1.5 into our formula and solve for moles.
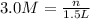
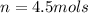
Now that we have moles, we can convert moles to grms by using the molar mass of LiBr.