Answer: The new volume is 544 L
Step-by-step explanation:
To calculate the final volume of the system, we use the equation given by Charles' Law. This law states that volume of the gas is directly proportional to the temperature of the gas at constant pressure.
Mathematically,
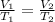
where,
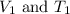 are the initial volume and temperature of the gas.
are the initial volume and temperature of the gas.
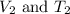 are the final volume and temperature of the gas.
are the final volume and temperature of the gas.
We are given:
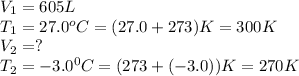
Putting values in above equation, we get:
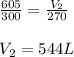
Thus the new volume is 544 L