Answer: Thus
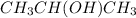 is a secondary alkanol.
is a secondary alkanol.
Step-by-step explanation:
Alkanol are compounds which contains carbons bonded by single bonds and contains hydroxy (-OH) as functional group.
Primary alkanol are those compounds which contain hydroxyl group attached a carbon which is further attached to a single carbon atom. Example:
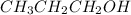 and
and
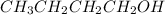
Secondary alkanol are those compounds which contain hydroxyl group attached to a carbon which is further attached to two more carbon atoms.Example:
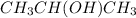
Tertiary alkanol are those compounds which contain hydroxyl group attached to a carbon which is further attached to three more carbon atoms. Example:
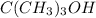
Thus
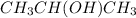 is a secondary alkanol.
is a secondary alkanol.