Answer:
The answer is 17.75%.
Step-by-step explanation:
We are given
 (ammonia) as a chemical formula. We want to find the percent composition of the formula.
(ammonia) as a chemical formula. We want to find the percent composition of the formula.
We are given the atomic masses of each element:
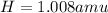 and
and
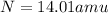 . Then, we need to find out how many atoms are present of each element in the compound.
. Then, we need to find out how many atoms are present of each element in the compound.
N has one atom (no subscript present). H has three atoms (subscript of 3 is present).
Now, we simply multiply the masses of each element by the number of atoms present of that element.
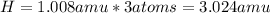
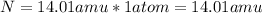
Then, we add up those products to get the mass of the formula.
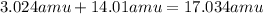
Finally, we divide the masses of each element by the mass of the formula to get the percentages of each element present in the compounds.
For hydrogen:
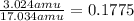
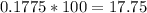
Hydrogen atoms account for 17.75% of the compound.
For nitrogen:
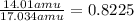
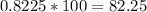
Nitrogen atoms account for 82.25% of the compound.
For an extra step, add up the percentages to make sure you get within 100 ± 0.01. Otherwise, you may have rounded incorrectly or miscalculated somewhere within your work.
Hope this helps!