Answer:
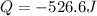
Step-by-step explanation:
Hello,
In this case, for the computation of the energy loss when the cooling process is carried out, we use the shown below equation:
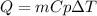
Whereas we need the mass, specific heat and change in temperature of iron within the process. Thus, the only value we need is the specific heat that is 0.444 J/(g°C), therefore, we compute the heat loss:
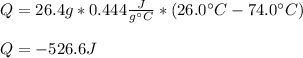
Negative sign points out the loss due to the cooling.
Regards.