Answer:
94.58 g of

Step-by-step explanation:
For this question we have to start with the reaction:
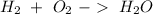
Now, we can balance the reaction:
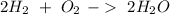
We have the amount of
 and the amount of
and the amount of
 . Therefore we have to find the limiting reactive, for this, we have to follow a few steps.
. Therefore we have to find the limiting reactive, for this, we have to follow a few steps.
1) Find the moles of each reactive, using the molar mass of each compound (
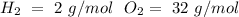 ).
).
2) Divide by the coefficient of each compound in the balanced reaction ("2" for
 and "1" for
and "1" for
 ).
).
Find the moles of each reactive
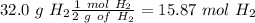
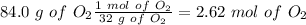
Divide by the coefficient
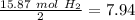
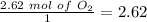
The smallest values are for
 , so hydrogen is the limiting reagent. Now, we can do the calculation for the amount of water:
, so hydrogen is the limiting reagent. Now, we can do the calculation for the amount of water:
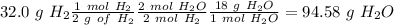
We have to remember that the molar ratio between
 and
and
 is 2:2 and the molar mass of
is 2:2 and the molar mass of
 is 18 g/mol.
is 18 g/mol.