Answer: A. The compound CuCl is 500 times less soluble in sea water than it is in pure water.
pure water.
Step-by-step explanation:
Any change in the equilibrium is studied on the basis of Le-Chatelier's principle.
This principle states that if there is any change in a equilibrium reaction, the equilibrium will shift in a direction so as to minimize the effect.
Thus when a common ion is introduced to an equilibrium reaction, the equilibrium will shift in a direction where the concentration of common ion is decreasing.
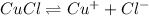
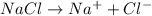
When common ion such as
 from NaCl in sea water is introduced to an equilibrium reaction, the equilibrium will shift in a direction where the concentration of common ion is decreasing i.e. in the left side and thus solubility of CuCl further decreases.
from NaCl in sea water is introduced to an equilibrium reaction, the equilibrium will shift in a direction where the concentration of common ion is decreasing i.e. in the left side and thus solubility of CuCl further decreases.