Answer:
2.29 L
Step-by-step explanation:
In this question we have to start with the chemical reaction:
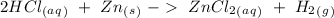
The reaction is already balanced. So, if we have an excess of HCl the compound that would limit the production of
 would be Zn. So, we have to follow a few steps:
would be Zn. So, we have to follow a few steps:
1) Convert from grams to moles (Using the atomic mass of Zn 65.38 g/mol).
2) Convert from moles of Zn to moles of
 (Using the molar mass 1 mol
(Using the molar mass 1 mol
 = 1 mol Zn).
= 1 mol Zn).
3) Convert from mol of
 to volume (Using the ideal gas equation PV=nRT).
to volume (Using the ideal gas equation PV=nRT).
First step:
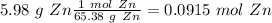
Second step:
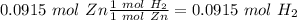
Third step:
We have to remember that R = 0.082
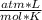 , so:
, so:
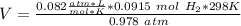
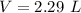
I hope it helps!