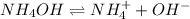Answer: a weak acid
Step-by-step explanation:
Oxidation reaction is defined as the reaction in which a substance looses its electrons. The oxidation state of the substance increases. Oxidizing agent is the substance which itself gets reduced by gaining electrons and thus oxidize others.
Reduction reaction is defined as the reaction in which a substance gains electrons. The oxidation state of the substance gets reduced. Reducing agent is the substance which itself gets oxidized by losing electrons and thus reduces others.
Weak acids are those substances which dissociate partially to give
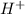 ions when dissolved in water.
ions when dissolved in water.
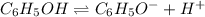
Weak bases are those substances which dissociate partially to give
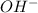 ions when dissolved in water.
ions when dissolved in water.