Answer:
The amount in grams of hydrogen gas produced is 0.551 grams
Step-by-step explanation:
The parameters given are;
Number of atoms of potassium, aₙ = 3.289 × 10²³ atoms
Chemical equation for the reaction is given as follows;
2K + 2H₂O
 KOH + H₂
KOH + H₂
Avogadro's number,
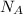 , regarding the number of molecules or atom per mole is given s follows;
, regarding the number of molecules or atom per mole is given s follows;
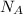 = 6.02 × 10²³ atoms/mole
= 6.02 × 10²³ atoms/mole
Therefore;
The number of moles of potassium present = 3.289 × 10²³/(6.02 × 10²³) = 0.546 moles
2 moles of potassium produces one mole of hydrogen gas, therefore;
1 moles of potassium produces 1/2 mole of hydrogen gas, and 0.546 moles of potassium will produce 0.546/2 moles of hydrogen which is 0.273 moles of hydrogen gas
The molar mass of hydrogen gas = 2.016 grams
Therefore, 0.273 moles will have a mass of 0.273×2.016 = 0.551 grams.
The amount in grams of hydrogen gas produced = 0.551 grams.