Answer: 323.61 g of
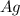 will be produced
will be produced
Step-by-step explanation:
The given balanced chemical reaction is :
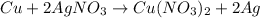
According to stoichiometry :
2 moles of
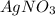 require 1 mole of
require 1 mole of
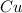
Thus 3.00 moles of
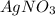 will require=
will require=
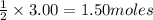 of
of
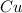
Thus
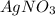 is the limiting reagent as it limits the formation of product.
is the limiting reagent as it limits the formation of product.
As 2 moles of
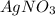 give = 2 moles of
give = 2 moles of
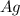
Thus 3.00 moles of
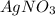 give =
give =
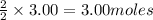 of
of
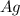
Mass of
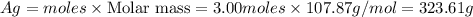
Thus 323.61 g of
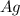 will be produced from the given moles of both reactants.
will be produced from the given moles of both reactants.