Answer:
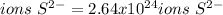
Step-by-step explanation:
Hello,
In this case, in one mole aluminium sulfide we can find three moles of sulfide ions, therefore, we can compute the ions by using the aforementioned molar ratio and the Avogadro's number as shown below:
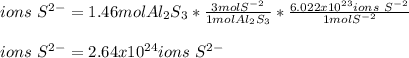
Best regards.