Answer:
Option B
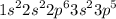
Step-by-step explanation:
In writing the electronic configuration for chlorine the first two electron will go in the 1s orbital.Since 1s can hold two electrons the next 2 electrons for chlorine go in the 2s orbital.The next 6 electrons will go in the 2p orbital.The p orbital can hold up to 6 electrons.We'll put six in the 2p orbital and then put the next 2 electron in the 3e.Since the 3s is now full.We'll move to 3p where we place remaining 5 electrons...
Thus,the electron configuration of chlorine(Cl) is
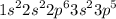
Hope this helps....
Good luck on your assignment...