Answer:
See figure 1
Step-by-step explanation:
For this question, we have to remember that in the lewis structures all atoms must have 8 electrons. And each atom would have a different value of valence electrons:
Carbon => 4
Oxygen=> 6
Hydrogen=> 1
Additionally, for the hybridizations we have to remember that:
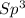 => 4 single bonds
=> 4 single bonds
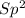 => 1 double bond
=> 1 double bond
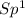 => 1 double bond
=> 1 double bond
With this in mind, the formaldehyde and formic acid would have
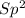 carbons and the ethanol an
carbons and the ethanol an
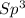 carbon.
carbon.
Finally, for the oxidation state. We have to remember that if we have more bonds with oxygen, we will have more oxidation. Therefore, the carbon that has more oxidation is the one in the formic acid (we have several bonds with oxygen).
See figure 1
I hope it helps!