Answer:
V₁ = 57.9 L.
Step-by-step explanation:
The initial volume can be found by calculating the work done on the system:
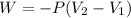
Where:
P: is the pressure = 0.557 atm
V₂: is the final volume = 55.1 L
V₁: is the initial volume =?
Now, the work can be calculated as follows:
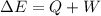
Where:
ΔE: is the internal energy of the system = -104.7 J
Q: is the heat absorbed = 51.0 J
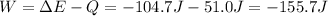
Hence, the initial volume is:
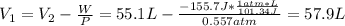
Therefore, the initial volume of the system is 57.9 L.
I hope it helps you!