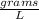Answer:
The density of CO₂ gas at 27 Celsius and 0.500 atm is 0.89
Step-by-step explanation:
An ideal gas is a theoretical gas that is considered to be composed of randomly moving point particles that do not interact with each other. Gases in general are ideal when they are at high temperatures and low pressures.
The pressure, P, the temperature, T, and the volume, V, of an ideal gas, are related by a simple formula called the ideal gas law:
P*V = n*R*T Equation (A)
where P is the gas pressure, V is the volume that occupies, T is its temperature, R is the ideal gas constant, and n is the number of moles of the gas.
Density allows you to measure the amount of mass in a given volume of a substance. So density is defined as the quotient between the mass of a body and the volume it occupies:
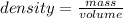
Being the molar mass of a substance the mass contained in one mole of said substance, the number of moles can be expressed as:
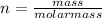 Equation (B)
Equation (B)
Replacing in Equation (A):
P*V=
 *R*T
*R*T
Solving to get the definition of density expressed in the equation, you get:
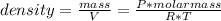
Being:
- P=0.500 atm
- Molar mass CO₂= 44 g/mole
- R= 0.082
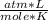
- T= 27 C= 300 K (being 0 C=273 K)
and replacing:
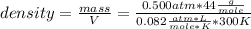
you get:
density= 0.89
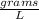
The density of CO₂ gas at 27 Celsius and 0.500 atm is 0.89