Answer:
The total internal energy change for the entire process is -0.94 kJ
Step-by-step explanation:
Process A to B is an isothermal process, therefore,
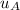 -
-
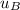 = 0
= 0
Process B to C
P = -mV + C
When P = 12, V = 0.12
When P = 4, V = 0.135
Therefore, we have;
12 = -m·0.12 + C
4 = -m·0.135 + C
Solving gives
m = 533.33
C = 76
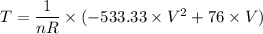
p₂ = p₁V₁/V₂ = 12*0.1/0.12 = 10 kPa
The work done = 0.5*(0.135 - 0.12)*(4 - 10.0) = -0.045 kJ = -45 J
For heat supplied
Assuming an approximate polytropic process, we have;
Work done = (p₃×v₃ - p₂×v₂)/(n - 1)
Which gives;
-45 = (4*0.135 - 10*0.12)/(n -1)
∴ n -1 = (4*0.135 - 10*0.12)/-45 = 14.67
n = 15.67
Q = W×(n - γ)/(γ - 1)
Q = -45*(15.67 - 1.4)/(1.4 - 1) = -1,605.375 J
u₃ - u₂ = Q + W = -1,605.375 J - 45 J = -1650 J = -1.65 kJ
For the constant pressure process D to C, we have;
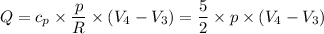
Q₄₋₃ = (0.1 - 0.135) * 4*5/2 = -0.35 kJ
W₄₋₃ = 4*(0.1 - 0.135) = -0.14 kJ
u₄ - u₃ = Q₄₋₃ + W₄₋₃ = -0.14 kJ + -0.35 kJ = -0.49 kJ
For the process D to A, we have a constant volume process
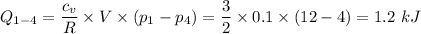
W₁₋₄ = 0 for constant volume process, therefore, u₁ - u₄ = 1.2 kJ
The total internal energy change Δ
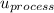 for the entire process is therefore;
for the entire process is therefore;
Δ
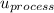 = u₂ - u₁ + u₃ - u₂ + u₄ - u₃ + u₁ - u₄ = 0 - 1.65 - 0.49 + 1.2 = -0.94 kJ.
= u₂ - u₁ + u₃ - u₂ + u₄ - u₃ + u₁ - u₄ = 0 - 1.65 - 0.49 + 1.2 = -0.94 kJ.