Answer:
See the explanation
Step-by-step explanation:
Inside this question we have different questions:
1. Draw all the chiral mono-chloro isomers of 3-methylpentane.
2. How many pairs of enantiomers are there for the following:
a. 2.3-dibromo-4-chloropentane
b. 2.4-dibromo-3-chloropentane
Lets start with the first one. In this question, we have to draw the products of a reaction called chlorination. In this reaction, we have a "Cl" atom into the molecule. Additionally, we only can add one "Cl" in each molecule (mono-chlorination), so we can have several options (see figure 1).
In the second question, first, we have to identify how many chiral carbons we have for both molecules. In a molecule, we have three chiral carbons, so we will have 8 enantiomers (
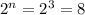 ) (see figure 2). In b molecule we have two chiral carbons we will have 4 enantiomers (
) (see figure 2). In b molecule we have two chiral carbons we will have 4 enantiomers (
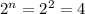 ) (see figure 3).
) (see figure 3).
I hope it helps!