Answer:
Rate of diffusion is same .
Step-by-step explanation:
As we know that Rate of the diffusion is directly proportional to the
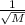 .They have same mass if there is same rate and similar condition therefore the mass of carbon (iv) oxide,propane and nitrogen (i) oxide will be similar.
.They have same mass if there is same rate and similar condition therefore the mass of carbon (iv) oxide,propane and nitrogen (i) oxide will be similar.
- The mass is directly proportional to the Rate of the diffusion.
- Therefore the rate of diffusion is similar in all carbon (iv) oxide,propane and nitrogen (i) oxide .