Answer:
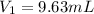
Step-by-step explanation:
Hello,
In this case, we work on a dilution process in which we can state that the moles remain the same after the dilution process. In such a way, we can write:
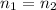
That in terms of molarities and volumes is:

Whereas
 is the initial molarity (3.00 M) of the stock solution,
is the initial molarity (3.00 M) of the stock solution,
 the molarity of the diluted solution (0.289 M),
the molarity of the diluted solution (0.289 M),
 the aliquot of the stock (concentrated) solution and
the aliquot of the stock (concentrated) solution and
 the volume of the diluted solution (100 mL), thus, we compute
the volume of the diluted solution (100 mL), thus, we compute
 as required:
as required:
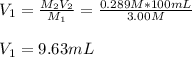
Best regards.