Answer: The final total volume of the gas is 20 L
Step-by-step explanation:
According to the Avogadro's law, the volume of gas is directly proportional to the number of moles of gas at same pressure and temperature. That means
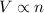
or,
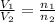
where,
 = initial volume of gas = 8.0 L
= initial volume of gas = 8.0 L
 = final volume of gas = ?
= final volume of gas = ?
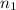 = initial moles of gas = 0.5 moles
= initial moles of gas = 0.5 moles
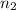 = final moles of gas = 0.5 + 0.75 = 1.25 moles
= final moles of gas = 0.5 + 0.75 = 1.25 moles
Putting in the values , we get:
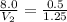
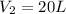
The final total volume of the gas is 20 L