Answer:
When solid oxalic acid dissolved in water, it dissociates , H+ - ions is formed, so acidic properties can be observed.
Step-by-step explanation:
When solid oxalic acid dissolved in water, it dissociates , H+ - ions is formed, so acidic properties can be observed.
C2H2O4 ---->
 +
+
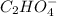
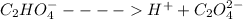
When oxalic acid is solid, there are no free moving H+ - ions , so acidic properties can not be observed.