Answer:
The final volume
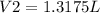
Step-by-step explanation:
between work ( w), pressure ( P ) and volume ( V ) is the following:
w=−PΔV
where,
ΔV=V2−V1
It was stated that the gas is expanding, then the work is done by the system and it is of a negative value .
Note that work, should be expressed in 1L⋅atm=101.3J
CHECK THE ATTACHMENT FOR DETAILED EXPLATION