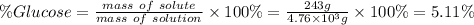Answer:
5.11 %
Step-by-step explanation:
Step 1: Convert the volume of water to millimeters
We will use the relationship 1 L = 1,000 mL.
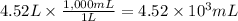
Step 2: Calculate the mass corresponding to 4.52 × 10³ mL of water
The density of water is 1.00 g/mL.
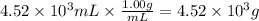
Step 3: Calculate the mass of the solution
The mass of the solute (glucose) is 243 g and the mass of the solvent (water) is 4.52 × 10³ g. The mass of the solution is:
m(solution) = m(solute) + m(solvent)
m(solution) = 243 g + 4.52 × 10³ g
m(solution) = 4.76 × 10³ g
Step 4: Calculate the mass percent of glucose in the solution