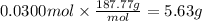Answer:
5.63 g
Step-by-step explanation:
Step 1: Write the balanced equation
CuBr₂(aq) + 2 AgCH₃CO₂(aq) ⇒ 2 AgBr(s) + Cu(CH₃CO₂)₂(aq)
Step 2: Calculate the reacting moles of copper (II) bromide
30.0 mL of 0.499 M CuBr₂ react. The reacting moles of CuBr₂ are:
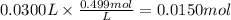
Step 3: Calculate the moles formed of silver (I) bromide
The molar ratio of CuBr₂ to AgBr is 1:2. The moles formed of AgBr are 2/1 × 0.0150 mol = 0.0300 mol.
Step 4: Calculate the mass corresponding to 0.0300 mol of AgBr
The molar mass of AgBr is 187.77 g/mol.