Answer:
a.
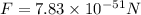
b.Attractive
Step-by-step explanation:
We are given that
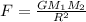
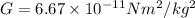
Mass of an electron,
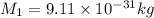
Mass of proton,
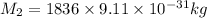
Distance between electron and proton,R=
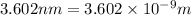
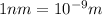
a.Substitute the values then we get
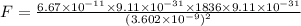
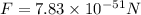
b.We know that like charges repel to each other and unlike charges attract to each other.
Proton and electron are unlike charges therefore, the force between proton and electron is attractive.