Answer:
6 atoms of carbon.
Step-by-step explanation:
Hello,
In this case, we can easily compute how many times the molar mass of the empirical formula is in the molecular formula by firstly computing it:
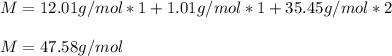
Then, we divide them:
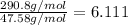
Which is approximately 6, for that reason, we are going to find six atoms of carbon in the molecule.
Regards.