Answer:
About 0.37 M.
Step-by-step explanation:
Recall that molarity is defined by moles of solute over total liters of solution:
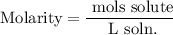
Determine the amount of moles of HCl in 40.0 grams. The molecular weight of HCl is 36.46 g/mol:
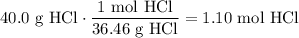
Therefore, the molarity of the 3.0 L solution is:

In conclusion, the molarity of the solution is about 0.37 M.