Answer:
About 0.0940 M.
Step-by-step explanation:
Recall that NaOH is a strong base, so it dissociates completely into Na⁺ and OH⁻ ions. Because the acid is monoprotic, we can represent it with HA. Thus, the reaction between HA and NaOH is:

Using the fact that it took 15.00 mL of NaOH to reach the endpoint, determine the number of HA that was reacted with:
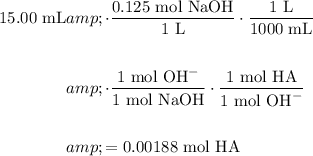
Therefore, the molarity of the original solution was:
![\displaystyle \left[ \text{HA}\right] = \frac{0.00188\text{ mol}}{20.00\text{ mL}} \cdot \frac{1000\text{ mL}}{1\text{ L}} = 0.0940\text{ M}](https://img.qammunity.org/2023/formulas/chemistry/high-school/ypk98bajqldif8a0y5drf1afzpfg0au4eq.png)
In conclusion, the molarity of the unknown acid is about 0.0940 M.