Answer:
Energy needed 1680kJ
Step-by-step explanation:
The quantity of heat required to raise the temperature of water to 100 degrees is expressed as
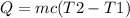
Given data
mass of water = 5kg
initial temperature T1= 20 °C
final temperature T2= 100 °C
Specific heat capacity of water= 4 200 J/Kg °C
