Answer:
0.5 kW
Step-by-step explanation:
The given parameters are;
Volume of tank = 1 m³
Pressure of air entering tank = 1 bar
Temperature of air = 27°C = 300.15 K
Temperature after heating = 477 °C = 750.15 K
V₂ = 1 m³
P₁V₁/T₁ = P₂V₂/T₂
P₁ = P₂
V₁ = T₁×V₂/T₂ = 300.15 * 1 /750.15 = 0.4 m³
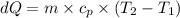
For ideal gas,
 = 5/2×R = 5/2*0.287 = 0.7175 kJ
= 5/2×R = 5/2*0.287 = 0.7175 kJ
PV = NKT
N = PV/(KT) = 100000×1/(750.15×1.38×10⁻²³)
N = 9.66×10²⁴
Number of moles of air = 9.66×10²⁴/(6.02×10²³) = 16.05 moles
The average mass of one mole of air = 28.8 g
Therefore, the total mass = 28.8*16.05 = 462.135 g = 0.46 kg
∴ dQ = 0.46*0.7175*(750.15 - 300.15) = 149.211 kJ
The power input required = The rate of heat transfer = 149.211/(60*5)
The power input required = 0.49737 kW ≈ 0.5 kW.