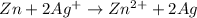Answer:
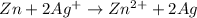
Step-by-step explanation:
Oxidation-reduction reaction or redox reaction is defined as the reaction in which oxidation and reduction reactions occur simultaneously and the number of electrons lost is equal to the number of electrons gained.
Oxidation reaction is defined as the reaction in which a substance looses its electrons. The oxidation state of the substance increases.
Anode :
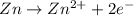
Reduction reaction is defined as the reaction in which a substance gains electrons. The oxidation state of the substance gets reduced.
Cathode :
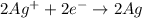
Thus the complete ionic equation will be :