Answer: 211.2 grams
Step-by-step explanation:
To calculate the number of moles for given molarity, we use the equation:
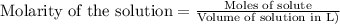 .....(1)
.....(1)
Molarity of
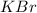 solution = 1.7 M
solution = 1.7 M
Volume of solution = 3.5 L
Putting values in equation 1, we get:
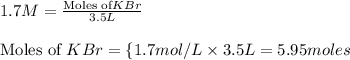
The balanced chemical reaction is:
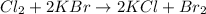
According to stoichiometry:
2 mole of KBr requires = 1 mole of

5.95 moles of KBr requires =
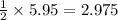 moles of
moles of

Mass of
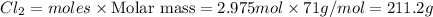
Thus 211.2 grams of chlorine gas are needed to react with 3.5 liters of a 1.7 molar potassium bromide solution